Abstract
Acute myeloid leukemia (AML) is characterized by excessive clonal proliferation of myeloid cells. Treatment options for relapsed/refractory AML include chemotherapy and/or allogeneic hematopoietic stem cell transplant (alloHSCT). However, the success rate for alloHSCT is limited with patients suffering from complications such as graft versus host disease. Therefore, an unmet need remains for developing new therapies for AML. CD33 and CD123 are highly expressed on AML blasts, with more than 70% expressing both, and have been targeted with CAR T cell therapies. Unfortunately, CD33 and CD123 are expressed on other noncancerous tissues such as hemopoietic stem cells. As a result, on-target off-tumor toxicities have limited chimeric antigen receptor (CAR) T cell therapies that target CD33 and CD123.
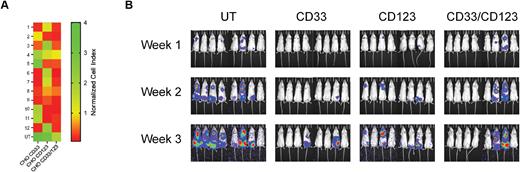
To overcome this, we developed bispecific human CD33/CD123 CAR T cells with an "AND” Logic gate. We produced 5 novel CD33 and 8 novel CD123 scFvs from monoclonal antibodies that bound CD33 and CD123 and activated T cells. Screening of monospecific CD33 or CD123 CAR T cells for cytotoxicity, cytokine production, and optimal proliferation was performed and based on these properties we selected 3 CD33 and 3 CD123 scFvs for CD33/CD123 bispecific CARs. The bispecific CARs had split intracellular signaling domains with 4-1BB on one scFv and CD3ζ on the other. Engineering T cells with split activation and costimulatory domains allows greater specificity and safer targeting of AML. To compare the cytotoxicity of the 12 bispecific CAR T cells, each were incubated with target cells expressing either CD33, CD123, or both CD33 and CD123. Cytotoxicity ranged from 1-4 on the normalized index, with most CAR T cell groups demonstrating higher levels of cytotoxicity with normalized index scores of 0-1 (Fig 1A). Bispecific CAR 1 showed high cytotoxicity against CD33 and CD33/CD123 target cells. Cytokine secretion of IFNγ, IL2, and IL6 correlated with cytotoxicity. Based on these results, we selected CAR 1 for further in vivo efficacy and safety testing. To evaluate the efficacy of CD33/CD123 bispecific CARs in vivo, we employed a xenogeneic human AML mouse model. We found rapid tumor expansion in the untransduced (UT) group by week two, while the monospecific and bispecific groups were better able to control the tumor (Fig 1B). Four mice in the CD33/CD123 group had lower bioluminescence imaging (BLI) than any mouse from the UT group at three weeks, while two mice given the bispecific CAR suffered breakthrough and had BLIs comparable to UT. Compared to the CD33 monospecific CAR, the bispecific group had similar BLIs with two mice from each group suffering tumor breakthrough. The CD33/CD123 mice also had similar BLIs to the group given the CD123 monospecific CAR. To evaluate the risk of on-target off-tumor effects with our bispecific CAR we i.v. injected 1x106 CAR T cells into CD34+ humanized NSG mice and found no significant percent change in body weight between mice given UT, CD33 monospecific, CD123 monospecific, or CD33/CD123 bispecific CAR T cells after 26 days. Blood was also collected from these mice at weeks 1-4 after CAR injection for complete blood cell count analysis and no differences were observed in white blood cell, lymphocyte, monocyte, hematocrit, or platelet counts between any of the treatment groups. We also examined the numbers of CD33+ or CD123+ in the blood at weeks 1-4 by flow cytometry and found no differences between the CD33/CD123 bispecific CAR group and either of the monospecific CAR groups suggesting low risk of toxicity. Overall, we demonstrate that optimizing the combination of potent scFvs and the costimulatory domain is important in developing a safer bispecific CAR T cell therapy. This meticulously developed CD33/CD123 bispecific CAR has therapeutic potential without increasing on-target off-target toxicity against refractory AML and will be further evaluated in clinical trials.
Disclosures
Maus:Arcellx: Consultancy; Astellas: Consultancy; AstraZeneca: Consultancy; Atara: Consultancy; Bayer: Consultancy; BMS: Consultancy; Cabaletta Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectis: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Consultancy, Research Funding; In8bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Intellia: Consultancy; GSK: Consultancy; Kite Pharma: Consultancy, Research Funding; Micromedicine/BendBio: Consultancy; Neximmune: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy, Research Funding; Oncternal: Consultancy, Current holder of stock options in a privately-held company; Sanofi: Consultancy; TCR2: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees; Tmunity: Consultancy; WindMIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Promab: Patents & Royalties: held by Massachusetts General Hospital; Novartis: Patents & Royalties: held by University of Pennsylvania; 2SeventyBio: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Allogene: Consultancy; Adaptimmune: Consultancy; Agenus: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal